Ozone(O3) is a blue gas, oxygen molecule containing three oxygen atoms. The usual oxygen we breathe contains two atoms.
Ozone is mainly found in the stratosphere and helps by trapping the dangerous ultraviolet radiation from reaching the troposphere. Without ozone, all life forms on Earth would not survive.
However, ozone has been thinning for the past years which has caused some undesirable effects particularly the poles. This decline in ozone is mostly attributed to chlouroflurocarbons produced by humans such as spraying aerosols and refrigerants.
CFCs (short for chlouroflurocarbons) make their way into the stratosphere and the UV light splits the chlorine from athe CFC molecule. The chlorine snatches and rips apart an ozone atom (see diagram below).
With time the ozone gets thinner and thinner and more ultraviolet radiation reaches the troposphere.
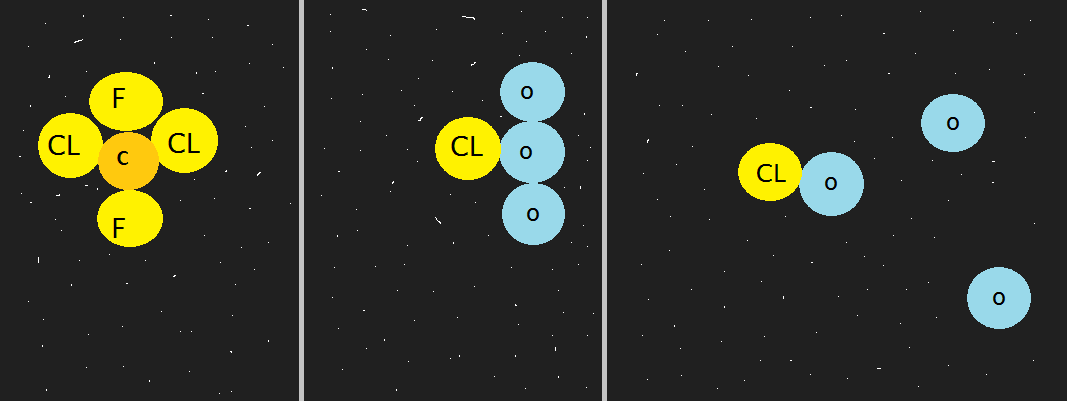
The most noticeable effect of ozone depletion is the thinning of ice sheets particularly in Antarctica. It has become to be known as the ozone hole(thin layer) along Antarctica. CFC concentration is high in cold conditions.
To combat CFC production and restore ozone, the Montreal Protocol was formed in 1989. The Montreal Protocol strive to restore ozone and the Ozone Hole over Antarctica by banning some CFC producing products. Estimates shows that ozone and the Ozone hole will recover in about 50 years.
